



Received: 02-Dec-2020, Manuscript No. GJFA-23-22839; Editor assigned: 07-Dec-2020, Pre QC No. GJFA-23-22839 (PQ); Reviewed: 21-Dec-2020, QC No. GJFA-23-22839; Revised: 14-Jun-2023, Manuscript No. GJFA-23-22839 (R); Published: 12-Jul-2023, DOI: 10.15651/2408-5464.23.10.001
This study aimed to determine the plankton abundance in selected brackish water fishponds in southern part of Surigao del Sur. A one-shot sampling was done on February 2019 at Lingig, Bislig and Hinatuan, Surigao del Sur, Philippines. This was conducted to identify which of the selected brackish water fishponds has the highest and least productivity of plankton. All the water quality parameters such as the temperature, dissolved oxygen, pH, ammonia, salinity and nitrite were within the optimal ranges for plankton productivity in all stations. A total of four groups of phytoplankton; Cyanophyceae, Bacillariophyceae, Dinophyceae and Chlorophyceae and six groups of zooplankton; Rotifera, Copepad, Cladoceran, Tintinnida (ciliate), Protozoa and Pelecypod larva were identified. The phytoplankton comprised of 33 genera in which Cyanophyceae was recorded the most abundant followed by Bacillariophyceae, Dinophycea and Chlorophyceae. The zooplankton comprised of 16 genera in which Copepod was recorded the most abundant followed by Rotifer, Pelecypod larva, Tintinnid and Protozoa. The most abundant phytoplankton in terms of location, Hinatuan recorded the most followed by Lingig and lastly, Bislig. Zooplankton was recorded most in Bislig followed by Hinatuan and Lingig. The most diverse phytoplankton was recorded in Lingig followed by Hinatuan and Bislig. Zooplankton was most diversed in Bislig followed by Lingig and least in Hinatuan. It was concluded that the phytoplankton groups provide the main support for earthen fishpond aquaculture compared to zooplankton group. It is further recommended to conduct follow up research with longer period to come up with more comprehensive data on the abundance of plankton community.
Plankton, Water quality, Physico-chemical parameters, Diversity
One of the main causes of water quality deterioration is fishpond management. Fishponds represent the most common type of standing water habitat in the Philippines and play an important role in the hydrological system and in the lives of most of the coastal dwellers. Fishponds are generally man-made shallow water bodies where water level, fish stock and to some extent also nutrient and fish food inputs are under the human control. Water quality determines the species optimal for culture under different environments. The overall production of a water body can easily be deduced from its primary productivity, which forms the backbone of the aquatic food chains. According to Battish, the plankton community is comprised either of the primary producers or phytoplankton and zooplankton, the secondary producers. It states further that the phytoplankton population represents the biological wealth of a water body, constituting a vital link in the food chain. The zooplankton forms the principal source of food for fish within the water body (Asha MSN et al., 2015).
The qualitative and quantitative abundance of plankton in a fishpond is of great importance in managing the successful aquaculture operations, as they vary from location to location and pond to pond within the same location even within similar ecological conditions. The physicochemical attributes of a water body are principle determinants of fish growth rates and development. It states further that fluctuations in physicochemical factors such as salinity, temperature, pH, the oxygen content in water or concentration of biogenesis increase ecological stress for many living organisms. For instance, water temperature impacts the growth and development of organisms and influence their mortality. Also, salinity has a significant impact on organisms because it requires them to adjust the saline concentrations in their bodies to the surrounding environment. Changes in salinity are the direct causes of some species disappearing to migrate to avoid unfavorable environmental conditions and other occurrences (Bir et al., 2015). Water pH can also have an impact on zooplankton; low pH causes reduction of zooplankton abundance, decreases biodiversity and losses some of the species, whereas alkaline conditions that accompany high primary production favors the growth and abundance of zooplankton. Oxygen dissolved in water, which is required for the survival of all aquatic organism mortality (Erondu and Solomon, 2017).
Biological pollution of the water resources has become a crucial concern today. Increasing human population and activities imposes great trouble on marine and freshwater resources. Among freshwater ponds are small but attributed with profuse aquatic macrophytes development. As disclosed by Md. Yeamin water quality, physicochemical and biological characteristics of water play a big role in plankton productivity as well as the biology of the cultured organisms and yields. Water quality determines the species optimal for culture under different environments. The present study will contribute information and hopefully help the owners and fishpond technician especially the selected brackish water fishpond in southern part of Surigao del Sur to apply the basic pond management principles that are designed to maintain water quality and reduce the incidence of disease (Harris and Vinobaba, 2012).
This study was conducted to assess the plankton diversity and abundance in fishpond to further determine its productivity and to hopefully recommend appropriate commodity to culture. Therefore, corrective measures should be put in place so as to prevent total ecological collapse (Malerba et al., 2012).
Description of the Study Area
The study area as shown in Figure 1 with the three selected stations namely; Lingig (station 1), Bislig (station 2) and Hinatuan (station 3). These selected stations were situated in the province of Surigao del Sur. Lingig geographical grid coordinates of 8.0873°N, 126.4148°E, Bislig 8.2101°N, 126.2848°E and Hinatuan 8.3837°N, 126.3715°E (Figure 1) (Hossain et al., 2007).
Field Assessment
The following were conducted in generating acquiring data: A coordination with the municipal officials before the actual sampling was conducted. The guidance of the assigned personnel from the Local Government Unit (LGU) and the owners of the selected brackish water fishpond in every station were considered very important in collecting the water samples (Paturej et al., 2017).
Data Gathering and Sampling Period
The sampling period in every station was conducted during daytime between 9:00 am-12:00 pm in the selected brackish water pond in southern part of Surigao del Sur (Chakraborty et al., 2011).
Water Sample Collection
The plankton can be found everywhere with the presence of water and sunlight. Sampling of pond water for plankton analyses was done on 100 liters filtered in plankton net from different areas of the pond. Preservation of the samples before analyses was done by addition of 5% buffered formalin in small plastic bottles and brought to laboratory for further analyses
Physico-chemical Parameters
Environmental parameters were determined by measuring the six factors below (Toseland et al., 2013). Field thermometer was used to distinguish the temperature, while salinometer was used to determine the salinity of the concentration of salt solutions in the water. The water quality tester set that contains chemicals were used to determine other parameters such as DO, pH, ammonia and nitrate (Usman et al., 2017).
Identification and Counting of Plankton
The water samples were brought to Surigao del Sur state university-Lianga campus biological laboratory which the samples are standby for 12 hours to let the plankton settle at the bottom of the sample bottle before analyses on a Sedgewick-rafter counting cell under a compound stereo microscope. Analyses involved transfer of 1 ml sub-sample from each of the samples to the Sedgewickrafter counting cells using pipette or dropper and identified as methods developed by Anna McGaraghan (Diana et al., 2022).
Data Analysis
In this study, species diversity measures used the following indices; Shannon-Weiner diversity index (H).
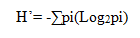
Where: pi is the proportional abundance of the ith species (ni/N)
Determination of relative abundance
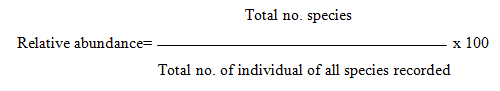
Physico-Chemical Parameters
During the sampling period, the highest water temperature was recorded in Lingig at 31.67°C followed by Hinatuan at 30.77°C and Bislig at 30.14°C with relative decrease of salinity from 8 ppt in Lingig, 6 ppt in Bislig and 4.34 ppt in Hinatuan. Temperature and salinity vary with the weather condition and tidal phase during the sampling. Bislig was recorded as the highest level of pH at 7.33 followed by Lingig at 7.2 and lowest in Hinatuan at 6.67. In terms of Dissolved Oxygen (DO), Bislig was also recorded to have the highest level at 8.0 ppm followed by Lingig at 5.83 ppm and Hinatuan at 5.5 ppm. While ammonia and nitrite level was recorded to have the same value in all station at 0 ppm and 0.1 ppm, respectively as shown in Table 1 (Dobiszewska et al., 2020).
| Parameters | Lingig | Bislig | Hinatuan |
|---|---|---|---|
| Temperature | 31.67°C | 30.14°C | 30.77°C |
| pH | 7.2 | 7.33 | 6.67 |
| Dissolved oxygen | 5.83 ppm | 8.0 ppm | 5.5 ppm |
| Ammonia | 0.00067 ppm | 0.00367 ppm | 0.00183 ppm |
| Salinity | 8 ppt | 6 ppt | 4.34 ppt |
| Nitrite | 0.1 ppm | 0.1 ppm | 0.1 ppm |
Table 1: Water quality parameters of selected brackish water pond in southern part of Surigao del Sur.
The variations in temperature, salinity, Dissolved Oxygen (DO), pH, ammonia and nitrite in the selected fishponds, the results on statistics were not significantly difference at 0.05 level of significance as shown in Table 2.
It implies that the physico-chemical parameters were within suitable range for plankton production in all sampling stations (Letelier et al., 2019).
| Parameters | Sum of squares | df | Mean of squares | CF | p-value | |
|---|---|---|---|---|---|---|
| Temperature | Between groups | 3.49416 | 2 | 1.74708 | 1.638 | 0.2706 |
| Within groups | 6.3992 | 6 | 1.06653 | |||
| Total | 9.89336 | 10 | - | |||
| Dissolved oxygen | Between groups | 1.74667 | 2 | 0.57333 | 0.2958 | 0.7542 |
| Within groups | 7.57333 | 6 | 1.26222 | |||
| Total | 9.32 | 10 | - | |||
| pH (acidity) | Between groups | 11.0556 | 2 | 5.52778 | 2.97 | 0.1269 |
| Within groups | 11.1667 | 6 | 1.86111 | |||
| Total | 22.2223 | 10 | - | |||
| Ammonia | Between groups | 0.72 | 2 | 0.36 | 4 | 0.07872 |
| Within groups | 0.54 | 6 | 0.09 | |||
| Total | 1.26 | 10 | - | |||
| Salinity | Between groups | 20.1867 | 2 | 10.0933 | 4.074 | 0.07627 |
| Within groups | 14.8645 | 6 | 2.47741 | |||
| Total | 35.0512 | 10 | - | |||
| Nitrite | Between groups | 0.72 | 2 | 0.36 | 4 | 0.07872 |
| Within groups | 0.54 | 6 | 0.09 | |||
| Total | 1.26 | 10 | - | |||
Table 2: ANOVA table for physico-chemical parameters.
As the result of physico-chemical parameters, Bislig was recorded to have the lowest temperature level which in turns results also to have the highest level of dissolved oxygen among the three sampling stations. The higher the temperature the lower the dissolved oxygen will be. Since the dissolved oxygen is probably the single most important water quality factor that pond managers need to understand. The water is unhealthy if the DO level is at below standard. This problem is often a consequence of too many fish, over fertilizing, overfeeding or excessive nutrients from livestock or fields as mentioned by Toseland et al. In the case of pH, based on the data recorded above, pH is on the ideal standard for the better growth of plankton. As cited by Chakraborty et al., the higher the pH the easier to form ammonia and vice versa. The ideal level for ammonia is 0 ppm. Since the ammonia level of all selected brackishwater pond was recorded to have less than 0 ppm, the water is on the ideal standard and the water is healthy. The water is toxic if the ammonia level is between 0.2 ppm-0.5 ppm. In this environment, the fish and other organism can be poisoned immediately and die. Nitrite can be an important nitrogen source, as it can be either released due to incomplete reduction of nitrate or taken up to supplement low nitrogen availability. According to (2012, nitrite level is most ideal at 0.3 ppm or lower. If the nitrite level in between 0.5 ppm-2.0 ppm, the water is not healthy. If the nitrite level is higher than 2 ppm, it is very dangerous to the production of plankton. And if the nitrite level is too high, it means that the nitrifying action is not performing well. This will indirectly cause the increase of the ammonia poisoning. Since it was recorded to have the same level of nitrite in all stations at 0.1 ppm, therefore the water is healthy and safe for the production of plankton (Mariakova et al., 2021).
The salinity act as a limiting factor in the distribution of living organism and its variation caused by dilution and evaporation is most likely to influence fauna in the coastal ecosystem. The distribution of phytoplankton along estuary gradients tends to favor cyanobacteria and chlorophytes in brackish waters. However, mid-to-high salinities in an estuary favor dinoflagellates and diatoms. Species diversity usually becomes very low at high salinities, but high salinities can be a lethal limit for many phytoplankton in estuaries as stated (Zegardlo et al., 2018).
Plankton Abundance and Diversity
The plankton population of Lingig, Bislig and Hinatuan was identified up to genus level and re-grouped into the various classes or groups as shown in Table 3 for phytoplankton and Table 4 for zooplankton. The total phytoplankton and zooplankton in three selected brackishwater pond in southern part of Surigao del Sur.
| Taxon | Genus | Stations | (%) | ||
|---|---|---|---|---|---|
| Phytoplankton | Lingig | Bislig | Hinatuan | Ra=N*100/Ns | |
| Cyanobacteria | Aphanizomenon | 3 | 36 | 41 | 31.01 |
| Cylindrospermopsis | 5 | 3 | 25 | 12.79 | |
| Gloeocapsa | 0 | 1 | 2 | 1.16 | |
| Microcystis | 16 | 7 | 12 | 13.57 | |
| Nodularia | 1 | 0 | 0 | 0.39 | |
| Oscillatoria | 5 | 1 | 25 | 12.02 | |
| Planktothrix | 7 | 29 | 31 | 25.97 | |
| Woronichinia | 0 | 2 | 6 | 3.1 | |
| 258 | 100 | ||||
| Diatom | Asterionellopsis | 0 | 1 | 0 | 0.69 |
| Bacteriastrum | 0 | 0 | 1 | 0.69 | |
| Cerataulina | 1 | 0 | 0 | 0.69 | |
| Coscinodiscus | 0 | 1 | 1 | 1.39 | |
| Cylindrotheca | 4 | 2 | 0 | 4.17 | |
| Guinardia | 2 | 1 | 0 | 2.08 | |
| Leptocylindrus | 0 | 0 | 1 | 0.69 | |
| Licmophora | 3 | 0 | 0 | 2.08 | |
| Lioloma | 0 | 1 | 0 | 0.69 | |
| Pleurosigma | 2 | 9 | 3 | 9.72 | |
| Proboscia | 31 | 5 | 6 | 29.17 | |
| Pseudo-nitzschia | 25 | 6 | 2 | 22.92 | |
| Rhizosolenia | 20 | 10 | 0 | 20.83 | |
| Skeletonema | 0 | 2 | 0 | 1.39 | |
| Thalassiosira | 1 | 0 | 0 | 0.69 | |
| Thalassiothrix | 2 | 0 | 0 | 1.39 | |
| Tropidineis | 1 | 0 | 0 | 0.69 | |
| 144 | 100 | ||||
| Dinoflagellate | Akashiwo | 1 | 0 | 0 | 9.09 |
| Amphinidium | 4 | 2 | 2 | 36.36 | |
| Ceratium | 2 | 0 | 1 | 18.18 | |
| Cochodinium | 0 | 0 | 1 | 0 | |
| Gyrodinium | 1 | 0 | 0 | 9.09 | |
| Karenia | 1 | 1 | 0 | 9.09 | |
| Prorocentrum | 2 | 3 | 1 | 18.18 | |
| 22 | 100 | ||||
| Chlorophyte | Radiococcus | 4 | 0 | 0 | 100 |
| 4 | |||||
Table 3: Comparison on abundance and composition (%) of phytoplankton of the brackish water fishpond in Lingig, Bislig and Hinatuan, Surigao del Sur.
| Taxon | Genus | Stations | (%) | ||
|---|---|---|---|---|---|
| Zooplankton | Lingig | Bislig | Hinatuan | Ra=N*100/Ns | |
| Rotifer | Colurella | 1 | 1 | 0 | 12.5 |
| Hexarthra | 0 | 1 | 0 | 0 | |
| Lecane | 4 | 6 | 4 | 50 | |
| Macrotrachela | 1 | 1 | 1 | 12.5 | |
| Phelodina | 1 | 0 | 0 | 12.5 | |
| Testudinella | 0 | 4 | 0 | 0 | |
| Trichocera | 1 | 0 | 0 | 12.5 | |
| 26 | 100 | ||||
| Copepod | Acartia | 1 | 6 | 7 | 100 |
| Copepod nauplius | 0 | 5 | 0 | 0 | |
| Eurytemora | 0 | 5 | 11 | 0 | |
| Harpacticoida | 0 | 1 | 0 | 0 | |
| 36 | 100 | ||||
| Cladoceran | Alona | 1 | 0 | 0 | 50 |
| Leptodora | 1 | 0 | 0 | 50 | |
| 2 | 100 | ||||
| Tintinnid (ciliate) | Tintinopsis fimbriata | 1 | 0 | 0 | 100 |
| 1 | 100 | ||||
| Protozoa | Arcella | 0 | 1 | 0 | |
| 1 | 100 | ||||
| Pelecypod larva | 0 | 4 | 0 | 100 | |
| 4 | 100 | ||||
Table 4: Comparison on abundance and composition (%) of zooplankton of the brackish water fishpond in Lingig, Bislig and Hinatuan, Surigao del Sur.
The phytoplankton population was recorded the most abundant in Hinatuan followed by Lingig and Bislig. On contrast, in the case of zooplankton abundance, the most abundant was recorded in Bislig followed by Hinatuan and Lingig (Figure 2).
The phytoplankton population was comprised of 33 genera of which falling into four major groups; Cyanophyceae, Bacillariophyceae, Dinophyceae and Chlorophyceae. Within these groups, Cyanophyceae was the most dominant with an average of 60.28% followed by Bacillariophyceae with 33.64%, Dinophyceae with 5.14% and Chlorophyceae with 0.94% (Figure 7). Cyanophyceae members were available in different habitats and most of the species were from fresh water. Since the salt concentration recorded in all the station were relatively low the more the Cyanophyceae can grow. Zooplankton population was comprised of 16 genera of which falling into six major groups; Rotifer, Copepod, Cladoceran, Tintinnid (ciliate), Protozoa and Pelecypond larva. Within these groups, Copepod was the most dominant with an average of 51.43% followed by Rotifer with 37.14%, Pelecypod larva with 5.71% while Tintinnid and Protozoa which had the same average of 1.43% as shown in Figure 8. Copepods were residing near the surface of the water, during the sampling most of the sample water were scooped near the body water surface of the pond; therefore, most of the identified zooplankton are Copepods. Copepods unlike other zooplankton have a much under adaptation to unfavorable climate and were also reported to be the abundant members of the zooplankton population (Table 5, Figures 3 and 4).
| Taxon | Stations | Total | % | ||
|---|---|---|---|---|---|
| Lingig | Bislig | Hinatuan | |||
| Phytoplankton | |||||
| Cyanophyceae | 37 | 79 | 142 | 258 | 60.28 |
| Bacillariophyceae | 92 | 38 | 14 | 144 | 33.64 |
| Dinophyceae | 11 | 6 | 5 | 22 | 5.14 |
| Chlorophyceae | 4 | 0 | 0 | 4 | 0.94 |
| 428 | 100 | ||||
| Zooplankton | |||||
| Rotifer | 8 | 13 | 5 | 26 | 37.14 |
| Copepod | 1 | 17 | 18 | 36 | 51.43 |
| Cladoceran | 2 | 0 | 0 | 2 | 2.86 |
| Tintinnid (Ciliate) | 1 | 0 | 0 | 1 | 1.43 |
| Protozoa | 0 | 1 | 0 | 1 | 1.43 |
| Pelecypon larva | 0 | 4 | 0 | 4 | 5.71 |
| 70 | 100 | ||||
Table 5: Comparison of percentage among different phytoplankton and zooplankton group of the brackish water fishpond in Lingig, Bislig and Hinatuan, Surigao del Sur.
The most diverse of phytoplankton was recorded in Lingig with an average of 1.19 followed by Hinatuan with 1.07 and Bislig with 1.01. In the case of zooplankton, the most diverse was recorded in Bislig with an average of 1.17 followed by Lingig with 1.13 and Hinatuan with 0.7 (Table 6 and Figure 5).
| Stations | Phytoplankton H=Pi*Ln (Pi) | Zooplankton H=Pi*Ln (Pi) |
|---|---|---|
| Lingig | 1.19 | 1.13 |
| Bislig | 1.01 | 1.17 |
| Hinatuan | 1.07 | 0.7 |
Table 6: Comparison of phytoplankton and zooplankton diversity in selected brackish water fishpond in Lingig, Bislig and Hinatuan, Surigao del Sur.
Based on the results, it was concluded that the environmental parameters such as temperature, salinity, DO, pH, ammonia and nitrite had influencing factor with the abundance and diversity of plankton. If disturbances continue, specifically the anthropogenic activities in nature, the parameters conditions will be affected, resulted to low productivity of plankton, not unless if these threats will be properly addressed. And if improper management of fishpond continues, it will also affect the abundance and diversity of phytoplankton and zooplankton. The phytoplankton groups provide the main support for earthen fishpond aquaculture compared to zooplankton group. Hinatuan supports a rich abundance of plankton followed by Bislig and Lingig. The abundance and diversity of plankton composition of Hinatuan may be attributed with the basic fishpond management. During sampling it was observed that the brackish water fishpond of Hinatuan was well managed than the other selected brackish water fishpond in southern part of Surigao del Sur.
It is therefore recommended to conduct similar study in multiple sampling period to determine the fluctuation rate on the abundance and diversity of the plankton. Also, seasonal changes of water quality parameters and effects on plankton production in the fishponds and extended monitoring are recommended in the future studies. Finally, an assessment of plankton abundance and diversity must be conducted along the water source of the fishpond or outside the fishpond compartment.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]