


Received: 18-Jul-2022, Manuscript No. GJCMB-22-72394; Editor assigned: 21-Jul-2022, Pre QC No. GJCMB-22-72394 (PQ); Reviewed: 04-Aug-2022, QC No. GJCMB-22-72394; Revised: 11-Aug-2022, Manuscript No. GJCMB-22-72394 (R); Published: 18-Aug-2022, DOI: 10.15651/gjcmb.22.10.008
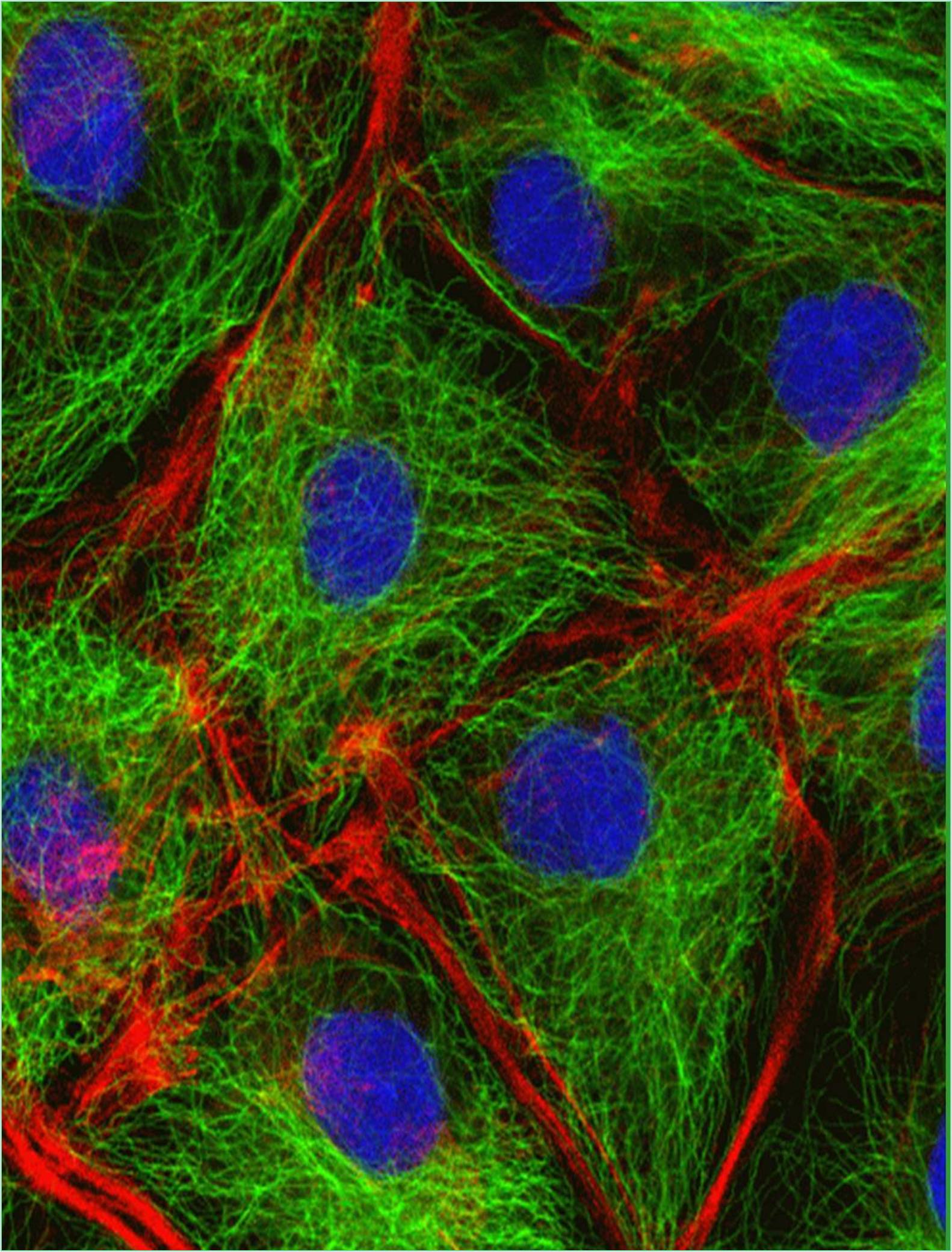
The molecular motors known as motor proteins are arguably the most interesting proteins that interact with the cytoskeleton. These extraordinary proteins attach to a polarized cytoskeletal thread and travel slowly along it using the energy produced by numerous cycles of ATP hydrolysis. Every eukaryotic cell contains dozens of distinct motor proteins that coexist. They differ in how they attach to filaments (actin or microtubules), how they move along the filament and what sort of “cargo” they are carrying. Numerous motor proteins transport membrane enclosed organelles to the correct sites in the cell including secretory vesicles, Golgi stacks and mitochondria. Various biological processes, including muscle contraction, ciliary beating and cell division are driven by the force created when other motor proteins cause cytoskeletal filaments to slide across one another.
The "head" portion or motor domain of the cytoskeletal motor proteins interacts with their filament tracks by ATP binding and hydrolysis. The proteins cycle between states in which they are tightly bound to their filament tracks and states in which they are free, coordinated with their cycle of nucleotide hydrolysis and conformational change. The motor protein and its associated cargo travel one step at a time along the filament through a mechanochemical cycle of filament binding, conformational alteration, filament release, conformational relaxation and filament rebinding. The motor domain (head) of the motor protein determines the identity of the track and the direction of movement along it whereas the motor protein's tail determines the identity of the cargo.
Kinesins and Dyneins
A motor protein that travels along microtubules is called kinesin. It was first discovered in the giant axon of the squid, where it walks toward the plus end of microtubules to transport membrane-enclosed organelles from the neural cell body to the axon terminal. With two heavy chains and two light chains per active motor, two globular head motor domains and an extended coiled-coil that promotes heavy chain dimerization, kinesin shares structural similarities with myosin II. Similar to myosin, kinesin belongs to a broad protein superfamily where the only thing in common is the motor domain. There are six different kinesins in the yeast Saccharomyces cerevisiae. There are roughly 40 kinesins in humans, compared to 16 in the nematode C. elegans.
The kinesin superfamily contains at least 10 families of proteins known as KRPs. The majority of them travel toward the plus end of the microtubule with the motor domain at the N-terminus of the heavy chain. A particularly intriguing family travels in the other way, toward the minus end of the microtubule with the motor domain at the C-terminus. The minus-end-directed microtubule motor family known as the dyneins is unrelated to the kinesin superfamily. They are made up of a vast and variable number of related light chains and two or three heavy chains (which include the motor domain).
Beyond their choice of various polymer tracks, the motor proteins in the myosin and kinesin superfamily’s display a remarkable array of motile characteristics. Most impressively, a single dimer of traditional kinesin moves in a highly processive manner, moving down a microtubule for hundreds of ATPase cycles without dissociating. In contrast, myosin II in skeletal muscle can only go a short distance along an actin filament before releasing. The varied biological roles of the motors depend on these distinctions. A tiny number of kinesin molecules must be very processive in order to move a mitochondrion all the way down a nerve cell axon.
The myosin in skeletal muscle never functions alone, instead it is a component of a vast array of myosin II molecules. Since efficient muscular contraction necessitates that each myosin head conduct its power stroke and then immediately get out of the way to avoid interfering with the actions of the other heads linked to the same actin filament here processivity would actually impair biological activity.
A kinesin dimer's two motor heads' mechanochemical cycles are coordinated with one another, preventing one from releasing until the other is ready to connect. Because of this cooperation, the motor protein may work hand in hand, never allowing the organelle cargo to diffuse off the microtubule track. The myosin heads of a myosin II dimer do not appear to be coordinated.